Featured
Latuda Release Date
Mitte 2005 hatte Dainippon Sumitomo Lurasidon an die US-amerikanische MSD Sharp Dohme auslizenziert. Showing results for Latuda Lurasidone Search instead.
 Generic Latuda Availability Drugs Com
Generic Latuda Availability Drugs Com
Die Vergütung der einzelnen Dosisstärken erfolgt entsprechend der Zulassung indikationsabhängig gemäss Fachinformation.
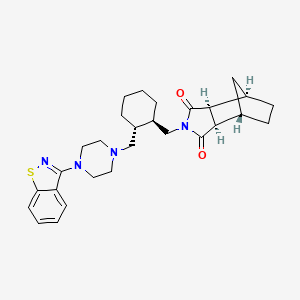
Latuda release date. LATUDA cpr pell 40 mg Lurasidone. Antimanic medications Second generation antipsychotic drugs. Greater with each lurasidone group than placebo and the benefit of the two lurasidone dose.
For the treatment of Schizophrenia the dosage range is from 150-750mg per day. Simply present the card to the pharmacist who will process the copay savings for you. The doctor will then adjust the dose according to how well the condition is being controlled.
Latuda can only be obtained with a prescription and in patients aged under 18 years it should be prescribed by an expert on mental illness in children. Danach führte Dainippon Sumitomo die Entwicklung wieder selbst durch. Latuda lurasidone is an atypical antipsychotic approved to treat bipolar depression and schizophrenia.
January 17 2019 909AM PT After a long wait generic Latuda lurasidone used to treat schizophrenia and bipolar disorder has been approved by the FDA and should be available in pharmacies soon according to manufacturer Lupin Pharmaceuticals. To get the LATUDA Copay Savings Card you simply register online here or over the phone at 1-855-5LATUDA1-855-552-8832. Efficacy and adverse effects of second-generation antipsychotics.
Latuda lurasidone is an atypical antipsychotic agent for the treatment of schizophrenia and bipolar depression. Latuda 20 mg und Latuda. Click here to upload a PDF.
7 Zeilen Latuda FDA Approval History. Generic Product News April 2019 April 20 2019. EFlow and eLete are registered trademarks of PARI Pharma GmbH.
Dainippon Sumitomo Pharma America Inc. The newly approved generic is indicated to treat overactive. Latuda 40 mg und Latuda 80 mg.
Bipolar major depression in adults. Was ist Latuda und wann wird es angewendet. The recommended starting dose is 37 mg once a day taken with food at around the same time each day.
Latuda is available as tablets to take by mouth. MAGNAIR is a registered trademark of PARI Pharma GmbH used under license. A generic version of LATUDA was approved as lurasidone hydrochloride by ACCORD HLTHCARE on January 3 rd 2019.
Yes First approved October 28 2010 Brand name. Anticipated release date. Merck zog sich jedoch schon 2006 wieder aus der Zusammenarbeit zurück.
Latuda wird zur Behandlung von Schizophrenie einer Erkrankung die zu den als Psychosen bezeichneten Krankheiten gehört verwendet. The cash price for a 30-day supply of brand-name Latuda is around 1500. Dry eyes eye inflammation Early 2019.
Latuda enthält als Wirkstoff Lurasidonhydrochlorid und wird vom Arzt oder von der Ärztin zur Behandlung der folgenden Krankheit verschrieben. Behandlung depressiver Episoden im Rahmen einer bipolaren Störung Typ 1. Then use the card when you fill your LATUDA prescriptions.
The initial dose for the treatment of Schizophrenia is 25mg and the maximum dose shouldnt extend 750mg daily. Latuda ist seit 2010 in den USA und seit März 2014 in der EU zugelassen. The FDA recently approved Camber Pharmaceuticals generic version of Detrol LA Tolterodine Tartrate ER capsules.
10 LIM CHF 12835. LATUDA is a registered trademark of Sumitomo Dainippon Pharma Co Ltd. Pharmacy Times April 2019 Mental Health Volume 85 Issue 4.
Latuda tablets had a market value of approximately 32 billion according to September 2018 data. LONHALA is a registered trademark and is a trademark of Sunovion Pharmaceuticals Inc. Veuillez patienter le système traite votre demande.
Schizophrenia bipolar disorder. The dose of the tablet with immediate release ranges from 25mg to 400mg while the extended-release is from 50mg to 400mg. Dabei handelt es sich um Störungen der Gehirnfunktion die das Denken.
Youll also get program updates when they become available. The release date of the generic formulation is currently unknown. Viibryd Teva Pharmaceuticals received FDA approval for their application for generic Viibryd vilazodone.
 Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
 Latuda Fda Prescribing Information Side Effects And Uses
Latuda Fda Prescribing Information Side Effects And Uses
 Latuda Vs Abilify Differences Similarities And Which Is Better For You
Latuda Vs Abilify Differences Similarities And Which Is Better For You
 Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
 Latuda Lawsuits Latuda Settlements Consumer Alert Now
Latuda Lawsuits Latuda Settlements Consumer Alert Now
Https Www Ema Europa Eu Documents Assessment Report Latuda Epar Public Assessment Report En Pdf
 Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
Latuda Lurasidone Hcl Tablets For Oral Administration Uses Dosage Side Effects Interactions Warning
 Lurasidone Tablets Fda Prescribing Information Side Effects And Uses
Lurasidone Tablets Fda Prescribing Information Side Effects And Uses
Latuda Full Prescribing Information Dosage Side Effects Mims Hong Kong
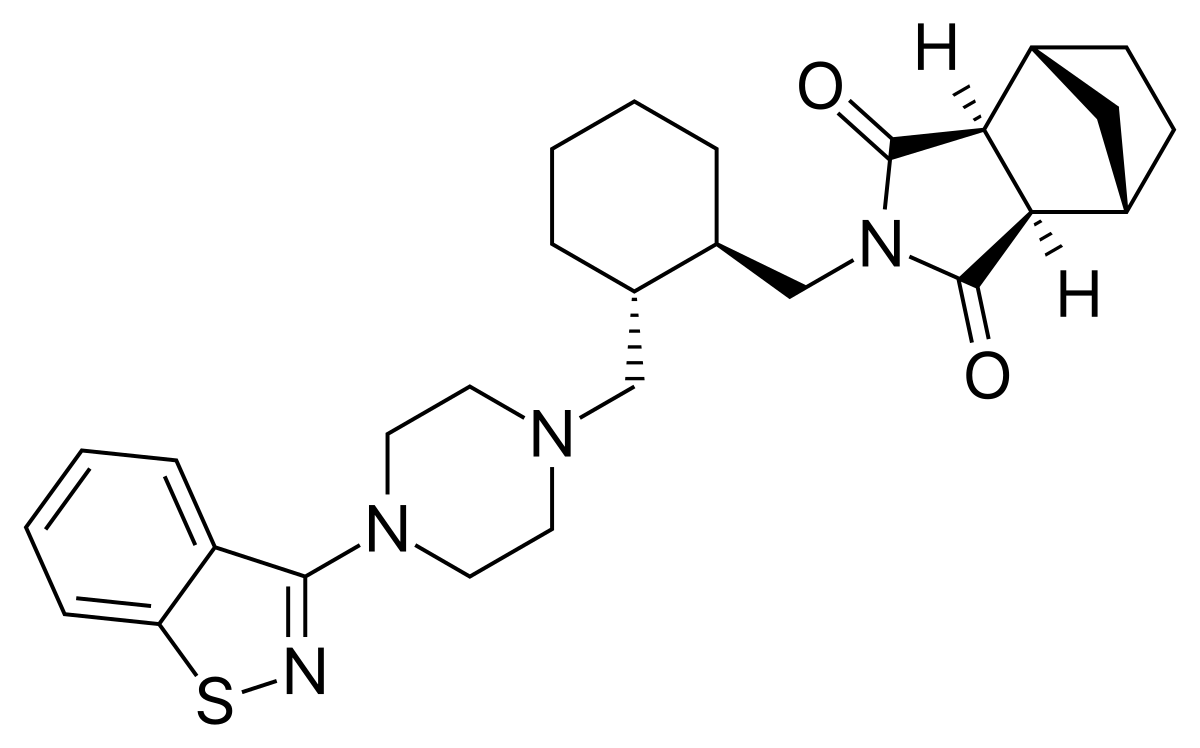


Comments
Post a Comment