Featured
Tecfidera Gi Side Effects
Flushing and stomach problems are the most common reactions especially at the start of therapy and may decrease over time. The incident rate of investigator-reported.
 Monomethyl Fumarate Has Better Gastrointestinal Tolerability Profile Compared With Dimethyl Fumarate Sciencedirect
Monomethyl Fumarate Has Better Gastrointestinal Tolerability Profile Compared With Dimethyl Fumarate Sciencedirect
A head-to-head trial of Vumerity versus Tecfidera found that the rate of treatment discontinuation with the new drug due to GI side effects was.

Tecfidera gi side effects. About TECFIDERA TECFIDERA is an oral therapy for relapsing forms of MS including relapsing-remitting MS the most common form of MS. Nausea vomiting diarrhea stomach pain or indigestion. A number of patients who start using Tecfidera experience undesirable gastrointestinal symptoms such as abdominal pain diarrhea nausea vomiting and indigestion dyspepsia as well as flushing.
TECFIDERA may cause serious side effects including. Common side effects of Tecfidera include flushing abdominal pain diarrhea and nausea. Know about the two common side effects that patients on Tecfidera may encounter.
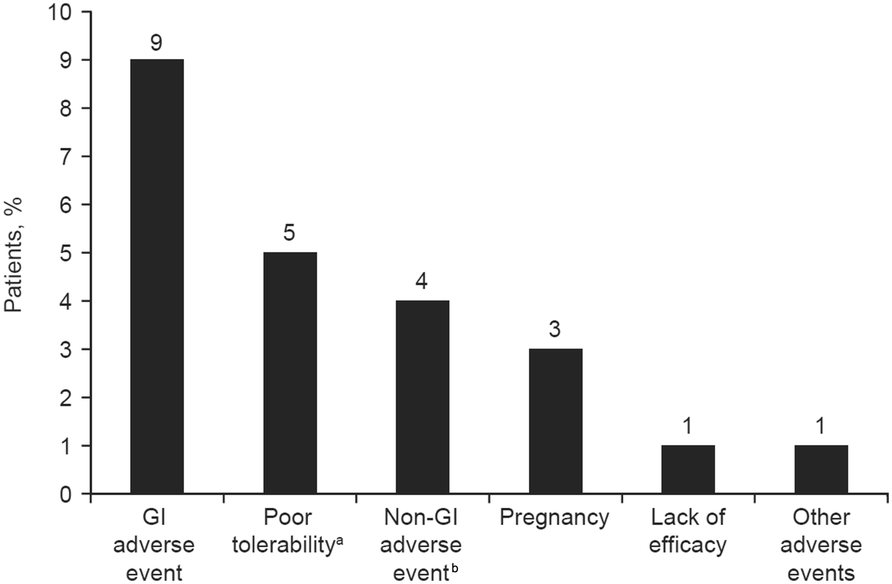
Of the remaining 25 12 reported either minimal or no adverse effects. The other 13 indicated significant side effects mainly nausea vomiting bloating andor diarrhea. These few important reminders for patients on the therapy can help.
For diarrhea the median. Flushing and stomach problems. Therapy discontinuation due to gastrointestinal side effects was 08 among Vumerity patients and 48 among those in the Tecfidera group.
406 for those on Tecfidera diarrhea. Flushing redness itching or rash. Prescribers and patients recognize the gastrointestinal side effects of Tecfidera as a significant hurdle to therapy that can delay titration to the target dose and although the gastrointestinal side effect profile reduces in severity over time it can still be bothersome enough to lead to discontinuation of Tecfidera.
If you have pre-existing stomach problems talk to your medical provider before taking Tecfidera. 13 rows TECFIDERA caused GI events eg nausea vomiting diarrhea abdominal pain and dyspepsia. What are the possible side effects of TECFIDERA.
In clinical trials 40 of patients who used Tecfidera experienced flushing and up to 18 experienced gastrointestinal GI symptoms. TECFIDERA caused GI events eg nausea vomiting diarrhea abdominal pain and dyspepsia. Serious side effects of Tecfidera include decreased white blood cell counts and increased liver enzymes.
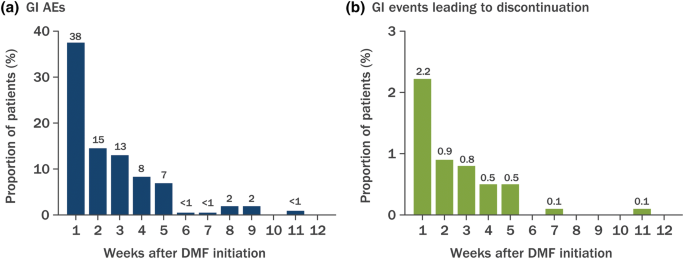
The adverse events resolved in 89 of patients and the median duration of the symptoms for overall GI events was 75 days. There is no adequate data on the developmental risk associated with the. The most common side effects of Vumerity include.
The incidence of GI events was higher early in the course of treatment primarily in month 1 and usually decreased over time in patients treated with TECFIDERA. TECFIDERA is currently approved in. The most common side effects in both treatment groups were flushing 328 for those using Vumerity.
Allergic reaction such as welts hives swelling of the face lips mouth or tongue or difficulty breathing PML a rare brain infection that usually leads to death or severe disability.
 Real World Characterization Of Dimethyl Fumarate Related Gastrointestinal Events In Multiple Sclerosis Management Strategies To Improve Persistence On Treatment And Patient Outcomes Springerlink
Real World Characterization Of Dimethyl Fumarate Related Gastrointestinal Events In Multiple Sclerosis Management Strategies To Improve Persistence On Treatment And Patient Outcomes Springerlink
Https Www Tecfidera Com Content Dam Commercial Tecfidera Pat En Us Pdf Questions When Considering Treatment Pdf
 Fewer Less Severe Gi Problems Seen In Rrms Patients Taking Vumerity In Phase 3 Trial
Fewer Less Severe Gi Problems Seen In Rrms Patients Taking Vumerity In Phase 3 Trial
 Gi Symptoms In Rrms Patients On Vumerity Fewer And Milder Than Tecfidera Phase 3 Trial Shows
Gi Symptoms In Rrms Patients On Vumerity Fewer And Milder Than Tecfidera Phase 3 Trial Shows
 Msology Managing The Tec Effect Msology
Msology Managing The Tec Effect Msology
 Tecfidera User Reviews For Multiple Sclerosis Drugs Com
Tecfidera User Reviews For Multiple Sclerosis Drugs Com
Biogen Files Tecfidera Follow Up In The Us Pmlive
 Tecfidera Up Against Bafiertam In Phase 1 Study Of Gi Tolerability Multiple Sclerosis News Today
Tecfidera Up Against Bafiertam In Phase 1 Study Of Gi Tolerability Multiple Sclerosis News Today
Dimethyl Fumarate Delayed Release Capsules
Tecfidera Successor S Side Effect Profile Boosts Biogen Pmlive
 New Ms Drug Vumerity Approved By Fda Everyday Health
New Ms Drug Vumerity Approved By Fda Everyday Health
Comments
Post a Comment