Featured
Rucaparib Package Insert
Giving enzalutamide and rucaparib may make patients live longer or prevent their cancer from growing or spreading for a longer time or both. During treatment with rucaparib events of myelosuppression anaemia neutropenia thrombocytopenia may be observed and are typically first observed after 8-10 weeks of treatment with rucaparib.
 Parp Inhibition For Ovarian Cancer Ppt Download
Parp Inhibition For Ovarian Cancer Ppt Download
Poly adenosine diphosphate ADP-ribose polymerase PARP inhibitors such as rucaparib fight prostate cancer by prevent tumor cells from repairing their DNA.

Rucaparib package insert. The updated RMP version 20 has also been submitted. Dosing and Administration 23 042018. 2016 -----INDICATIONS AND USAGE----- RUBRACA is a poly ADP-ribose polymerase PARP inhibitor indicated as monotherapy for the treatment of patients with deleterious.
Die Summenformel lautet C 19 H 18 FN 3 O. In addition the applicant took the opportunity to propose the move of one paragra ph from section 44 to 51 in the SmPC for consistency with other SmPC agents in. RUBRACA rucaparib tablets for oral use.
Niraparib is approved for the maintenance treatment of adult patients with recurrent ovarian cancer following a complete or partial response to platinum-based chemotherapy. Das Molekulargewicht beträgt 3307 gmol. This press release contains forward-looking statements as defined under the Private Securities Litigation Reform Act of 1995 about the potential of Rubraca rucaparib for the treatment of adult patients with deleterious BRCA mutation germline andor somatic-associated metastatic castration-resistant prostate cancer mCRPC who have been treated with androgen receptor-directed.
Each 200 mg tablet contains 344 mg rucaparib camsylate equivalent to 200 mg rucaparib free base. Warnings and Precautions 51 042018-----INDICATIONS AND USAGE-----. Accessed August 31 2020.
It may also help doctors learn if a mutation in any of the homologous. Each 250 mg tablet contains 430 mg rucaparib camsylate equivalent to 250 mg rucaparib. In vitro studies have shown that rucaparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and.
Complete blood count testing prior to starting treatment with Rubraca and. Rucaparib camsylate is a white to pale yellow powder. Rucaparib is an inhibitor of poly ADP-ribose polymerase PARP enzymes including PARP-1 PARP-2 and PARP-3 which when uninhibited play a role in DNA repair.
Rucaparib is a PARP inhibitor. Rucaparib shows pH-independent low solubility of approximately 1 mgmL across the physiological pH range. This enhances the accumulation of DNA strand breaks promotes genomic.
Rubraca rucaparib tablets contain rucaparib camsylate as the active ingredient. Hmm approval in. PARP inhibitors have been in clinical trials for several years.
The Package Leaflet is also updated in accordance. Upon administration rucaparib selectively binds to PARP1 2 and 3 and inhibits PARP-mediated DNA repair. Rucaparib tablets for oral use Initial US.
Rucaparib enthält vier zyklische Teilelemente darunter ein Benzol und ein Fluor-Benzen. Formulated into a tablet for oral use. Rucaparib ist ein antineoplastischer Arzneistoff aus der Klasse der PARP-Inhibitoren zur Behandlung des Ovarialkarzinoms.
The indication for rucaparib is for patients who have been treated with two or more prior. These reactions are manageable with routine medical treatment andor dose adjustment for more severe cases. Rucaparib is an orally bioavailable tricyclic indole and inhibitor of polyADP-ribose polymerases PARPs 1 PARP1 2 PARP2 and 3 PARP3 with potential chemoradiosensitizing and antineoplastic activities.
The NCCN Drugs and Biologics Compendium NCCN Compendium. 2016 -----RECENT MAJOR CHANGES-----Indications and Usage 11 12 042018. According to the package insert for niraparib CBCs should be assessed weekly during the first month of therapy monthly for the next 11 months and.
Program Step Therapy Rubraca rucaparib Change Control 102019 New program 102020.

Https Www Ema Europa Eu En Documents Product Information Rubraca Epar Product Information En Pdf
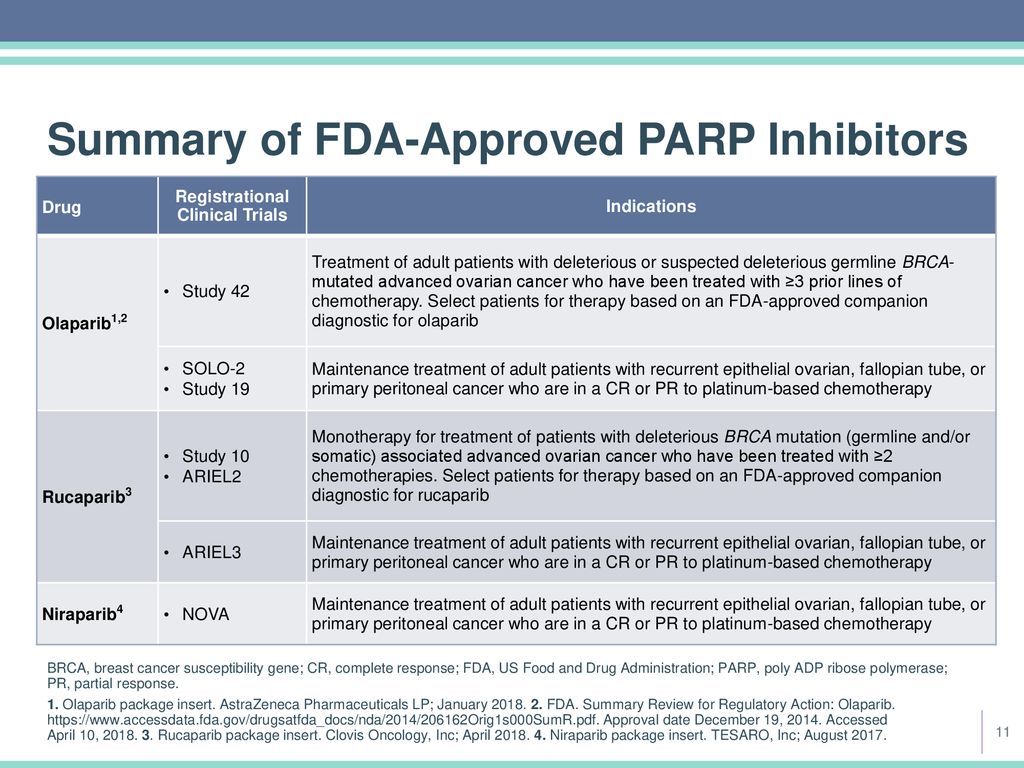 Parp Inhibition For Ovarian Cancer Ppt Download
Parp Inhibition For Ovarian Cancer Ppt Download
Https Www Accessdata Fda Gov Drugsatfda Docs Nda 2016 209115orig1s000multidiscipliner Pdf
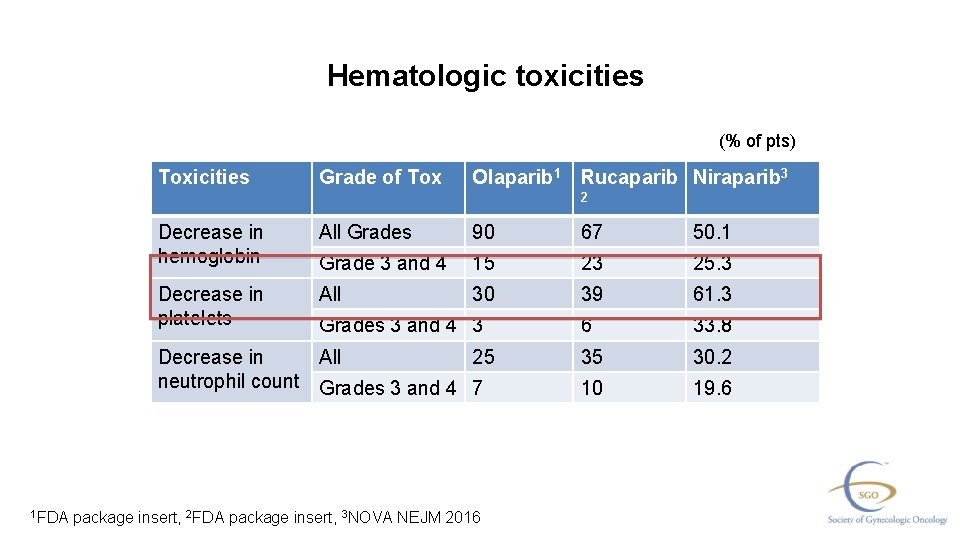 Please Note These Are The Actual Videorecorded Proceedings
Please Note These Are The Actual Videorecorded Proceedings
 Manufacturer Clovis Oncology Inc Fda Approval Date December Ppt Video Online Download
Manufacturer Clovis Oncology Inc Fda Approval Date December Ppt Video Online Download
 Frequently Asked Questions About Rubraca Rucaparib Cancerconnect
Frequently Asked Questions About Rubraca Rucaparib Cancerconnect
Https Www Accessdata Fda Gov Drugsatfda Docs Nda 2016 209115orig1s000otherr Pdf
Https Www Accessdata Fda Gov Drugsatfda Docs Nda 2016 209115orig1s000multidiscipliner Pdf
 Rucaparib Rubraca Oncology Nurse Advisor
Rucaparib Rubraca Oncology Nurse Advisor
Https Www Clovisoncology Com Media 1094 Rubraca Prescribing Info Pdf
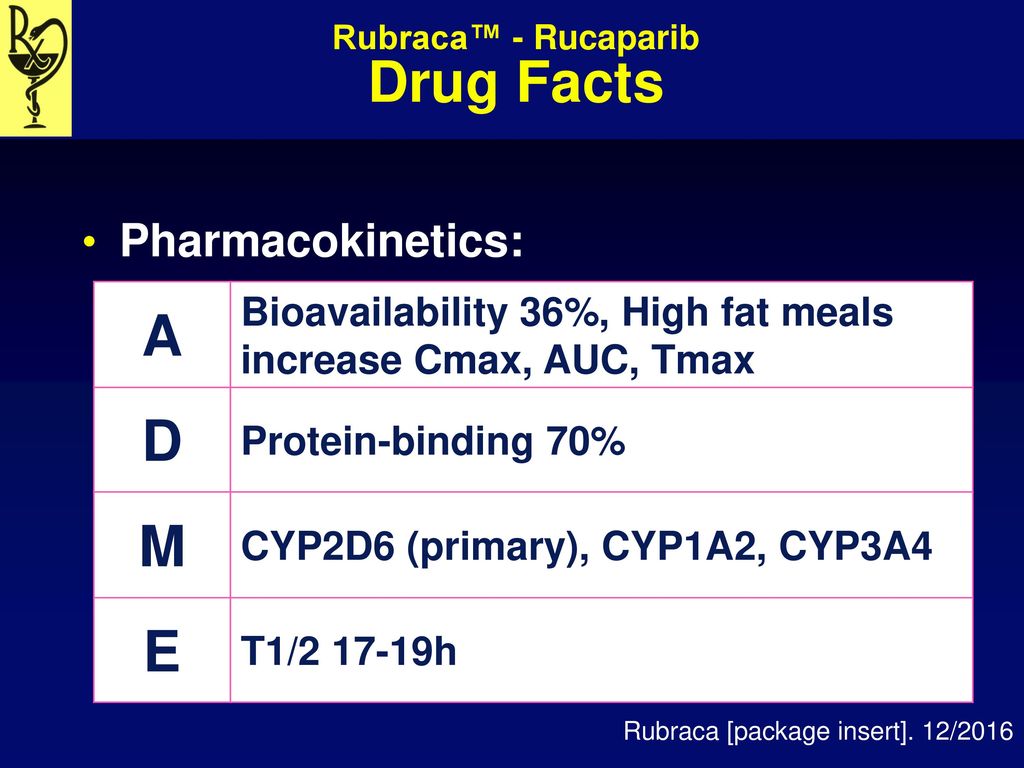 Manufacturer Clovis Oncology Inc Fda Approval Date December Ppt Video Online Download
Manufacturer Clovis Oncology Inc Fda Approval Date December Ppt Video Online Download
Comments
Post a Comment