Featured
Biofire Stool Test
The BioFire FilmArray Gastrointestinal Panel is an FDA-cleared assay for testing Cary-Blair-preserved stool. Compared to conventional testing methods the FGP costs 40 more or about 180 CDN per test.
 Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
Stool tests can be a valuable diagnostic tool to assess your digestive tract if you have signs and symptoms of gut infections such as.

Biofire stool test. Principle of the Procedure The BioFire GI Panel pouch is a closed system disposable that houses all the chemistry required to isolate amplify and detect nucleic acid from multiple gastrointestinal pathogens within a single stool specimen. Frequent bowel movements diarrhea bloody diarrhea or mucous in your stool. The BioFire GI Panel platform has demonstrated a sensitivity of.
A leading blood test laboratory of India Dr Lal PathLabs now makes it easy and convenient for patients to check their lab test results online with just a couple of clicks. The Gastrointestinal Profile Stool PCR 183480 from LabCorp is a highly sensitive and specific multiplexed nucleic acid test intended for the simultaneous qualitative detection and identification of nucleic acids from multiple bacteria viruses and parasites obtained from individuals with signs andor symptoms of gastrointestinal infection. Assays were assessed only for the targets common among all three.
The BioFire FilmArray Gastrointestinal Panel GIP was implemented to replace traditional stool culture and enzyme immunoassay EIA testing for stool pathogens. The manufacturer claims a sensitivity of 985 and specificity of 992 for this test and states that results are available within one hour of testing. Rotavirus A and Sapovirus I II IV and V BioFire 2019a.
Coli STEC norovirus and rotavirus. The FilmArray Gastrointestinal GI PCR Panel BioFire Diagnostics Salt Lake City UT USA received FDA approval in 2014 and can identify 22 bacterial viral and protozoan pathogens associated with gastroenteritis directly from stool samples within approximately 1 h 16. Namely Campylobacter Salmonella Shigella Shiga toxin-producing E.
The BioFire FilmArray Gastrointestinal Panel FGP is a multiplex polymerase chain reaction test that can simultaneously test for 22 different viruses bacteria and parasites with excellent sensitivity and specificity with a turnaround time of 1 hour. The BioFire FilmArray System is the new standard for syndromic infectious disease testing. The GI pathogen panel stool test is a highly-specialized laboratory test that is capable of rapidly detecting 22 different kinds of bacteria viruses and other microorganisms responsible for causing disease in the GI tract.
Severe abdominal pain and bloating. Rectal swabs endoscopy stool aspirates vomitus or other transport media are not FDA approved for this assay and will be rejected if received. What Are Stool Tests Used For.
The BioFire FilmArray GI Panel is a 510 k-cleared in vitro diagnostic IVD test capable of simultaneous detection and identification of nucleic acids from multiple bacteria n13 viruses n5 parasites n4 directly from stool samples collected in Cary Blair transport media obtained from individuals with signs andor symptoms of gastrointestinal GI infection. A total of 152 stool specimens were tested using three platforms. BioFire Gastrointestinal GI Panel Testing Page 2 of 17 FLM1-MKT-0071-03.
Verigene Enteric Pathogens Test Verigene Biofire FilmArray Gastrointestinal Panel Biofire and Luminex xTAG Gastrointestinal Pathogen Panel Luminex. Prospective samples230 were evaluated. A performance verification study of the FilmArray Gastrointestinal Panel was completed at Mayo Clinic Rochester Minnesota1 Five hundred clinical stool specimens retrospectivestored samples270.
With integrated sample preparation amplification detection a. However BioFire notes that the test has not been evaluated for immunocompromised patients BioFire 2019a. This test helps a physician to diagnose the cause of abdominal symptoms most commonly diarrhea more accurately and efficiently than traditional testing.
Results were compared to a reference. The performance of this test has only been validated with 200µL of human stool collected in Cary Blair Transport medium. This GI profile is a multiplexed nucleic acid test intended for the simultaneous qualitative detection and identification of nucleic acids from multiple bacteria viruses and parasites directly from stool samples in Cary-Blair transport media obtained from individuals with signs andor symptoms of.
STOOL PATHOGENS BIOFIRE PCR - Springfield Hospital Laboratory Test Catalog. The rigid plastic component fitment of the BioFire GI Panel pouch. Other unexplained digestive and non-digestive symptoms.
 Diarrhea Causes Diagnosis Biofire Diagnostics
Diarrhea Causes Diagnosis Biofire Diagnostics
 Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
 New Study Suggests Copan Fecalswab Works Well With Biofire Filmarray Gastrointestinal Gi Panel
New Study Suggests Copan Fecalswab Works Well With Biofire Filmarray Gastrointestinal Gi Panel
 How To Use Copan S Fecalswab With The Biofire Filmarray Gi Panel Youtube
How To Use Copan S Fecalswab With The Biofire Filmarray Gi Panel Youtube
Https Www Biofiredx Com Wp Content Uploads 2016 03 Is Mrkt Prt 0234 07 Filmarray Gastrointestinal Panel Information Sheet Pdf
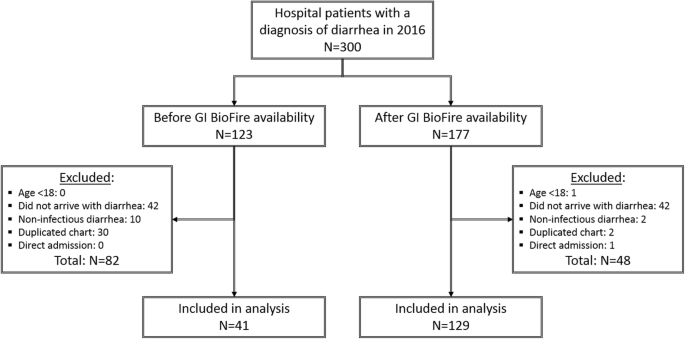 Use Of Biofire Filmarray Gastrointestinal Pcr Panel Associated With Reductions In Antibiotic Use Time To Optimal Antibiotics And Length Of Stay Bmc Gastroenterology Full Text
Use Of Biofire Filmarray Gastrointestinal Pcr Panel Associated With Reductions In Antibiotic Use Time To Optimal Antibiotics And Length Of Stay Bmc Gastroenterology Full Text
Https Www Ijidonline Com Article S1201 9712 17 30304 1 Pdf
Https Www Biofiredx Com Wp Content Uploads 2018 05 Flm1 Mkt 0071 Filmarray Gi Procedure Clsi Format Pdf
 Practice Based Learning Biofire Filmarray Gi Panel In Acute Gastroenteritis Pediatric Focus
Practice Based Learning Biofire Filmarray Gi Panel In Acute Gastroenteritis Pediatric Focus
 Biofire Gi Pathogen Panel Ijamsville Germantown Glenn Dale Olney Md
Biofire Gi Pathogen Panel Ijamsville Germantown Glenn Dale Olney Md
Https Www Labcorp Com Assets 17169
 Pdf Evaluation Of The Biofire Film Array Gastrointestinal Panel Test For The Screening Of Pathogens In Stools
Pdf Evaluation Of The Biofire Film Array Gastrointestinal Panel Test For The Screening Of Pathogens In Stools
 Biofire Filmarray Multiplex Pcr System Biomerieux
Biofire Filmarray Multiplex Pcr System Biomerieux
 Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
Gastrointestinal Gi Pathogen Panel Biofire Diagnostics
Comments
Post a Comment